Sleep
A Link Between Sleep Deprivation and Alzheimer's Disease?
Does lack of sleep increase the risk of Alzheimer's disease (AD)?
Posted December 26, 2023 Reviewed by Ray Parker
Key points
- Accumulation of beta-amyloid and tau proteins are the hallmarks of Alzheimer’s disease (AD).
- Sleep deprivation may lead to an accumulation of these proteins and an increased risk of developing AD.
- Research indicates sleep is essential in clearing these proteins from the brain.
Sleep knits up the raveled sleeve of care, or so says the Bard of Avon (Shakespeare, Macbeth, Act 2 Scene 2). A good night’s sleep does tend to make us feel better. Its opposite, sleep deprivation (SD), can make getting through the next day significantly more difficult.
New research suggests that SD can also increase the risk of Alzheimer’s disease (AD) due to its effects on the accumulation of plaque in the brain.
Plaques and tangles
Scientists know what the neurological markers are in AD. Maiken Nedergaard puts it succinctly, saying,
“Essentially all neurodegenerative diseases are associated with mis-accumulation of cellular waste products…tau and βeta-amyloid [proteins] can accumulate as stable aggregates that are neurotoxic in conditions such as Alzheimer’s disease” (Nedergaard, 2013).
Beta-amyloid proteins form plaques, which are clumps of pieces of these proteins that accumulate outside of the neurons of the brain. They surround the neurons and are difficult for the brain to eliminate.
Small clumps of beta-amyloids can block the messages that cells normally send to one another, trigger inflammation, and ultimately have a toxic effect on brain cells. Beta-amyloid proteins interact with tau proteins that make up the skeletal structure of the neurons, causing them to clump together inside the cells, forming what are called tangles and causing the internal structure of the neuron to fall apart.
The presence of plaques outside of the neurons in the brain and tangles inside the cells are the hallmarks of Alzheimer’s disease.
Historically, a definitive diagnosis of AD required an autopsy and examination of the brain under a microscope to see the accumulation of plaques and neurofibrillary tangles. Until recently, doctors had to rely on changes in behavior brought about by the accumulation of plaques and tangles in the brain. Changes in memory, cognitive ability, motor function, language, and emotional response might lead to a suspicion of the disorder. Still, a definitive diagnosis of AD has to wait until the death of the person suffering from dementia to happen.
The search for a biomarker
The search is on for what are called biomarkers for AD. A biomarker is a physical and clearly defined characteristic that can be measured as an indicator of normal or pathological processes (Califf, 2018). The earlier the biomarker “marks” the potential for disease, the stronger the possibility for treatment and early intervention.
The search is on for a way to scan the living and functioning brain to see the buildup of abnormal proteins that would mark AD. New developments in positron emission tomography or PET scans, primarily used in current research settings, might allow the detection of amyloid and tau proteins in the living brain. It is possible to detect and measure both amyloid and tau proteins in the cerebrospinal fluid, and new blood tests are in the works that will allow the detection of amyloids and tau in the bloodstream.
However, these new tests and markers are not yet ready for use by physicians. Some new research may point to a behavioral test that might provide very early hints about the risk of developing Alzheimer’s disease.
Researchers have shown that, in mice, beta-amyloids accumulate during wakefulness and that sleep is associated with a significant increase in the metabolism and clearance of beta-amyloid proteins from the brain (Xie, 2013). Other studies have shown that, again, in rodents, sleep deprivation can lead to significant increases in beta-amyloids (Kang et al., 2009).
Recently, a similar association between sleep and protein accumulation and metabolism in the brain has been noted in humans. Kojori, Wang, Wiers, et al. (2018) examined the brains of 20 healthy adults after both normal, uninterrupted sleep and after a night of sleep deprivation using PET scans to measure the accumulation and elimination of beta-amyloids in these two conditions.
Sleep and clearing plaques from the brain
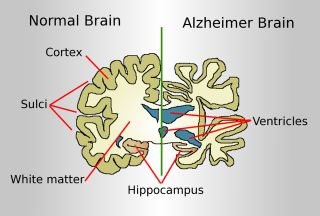
They found that one night of sleep deprivation resulted in significantly higher levels of beta-amyloid accumulation compared to levels seen after normal and uninterrupted sleep. These higher levels of protein accumulation were seen in the right hemisphere in regions of the brain known to be affected by AD, and the main behavioral symptom of AD is loss of memory. These regions were the hippocampus, parahippocampus, and thalamus.
They also found a significant inverse correlation between the number of hours of sleep and the accumulation of beta-amyloids in these regions. The lower the number of hours of sleep obtained, the higher the accumulation of these plaque proteins.
They concluded that impaired sleep was a risk factor for the development of AD. Presumably, the metabolism and clearance of beta-amyloid proteins that normally happen during sleep were decreased with lack of sleep. However, because their measure of the clearance of these proteins was indirect and because direct measurement of the removal of these proteins had not yet been accomplished in huma at the time of their study at the time of their study, this conclusion remains to be tested.
References
Califf, R.M. (2018) Biomarker definitions and their applications. Experimental Biology and Medicine, 243, 213–221. DOI: 10.1177/1535370217750088
Mayo Clinic Staff, (2022) Diagnosing Alzheimer's: How Alzheimer's is diagnosed https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/in-depth/alzheimers/art-20048075
Nedergaard, M. (2013). Garbage truck of the brain. Science, 340(6140), 1529-1530. DOI:10.1126/science.1240514
Shokri-Kojori, E. Wang, G-J Wiers, C.E. Sukru B. Demiral, S.B., Guoa, M., Kim,S.W., Lindgren, E., Ramirez, V., Zehra,,A., Freeman, C., Miller, G., Manza, P., Srivastava, T.,De Santi, S. Tomasi, D., Benveniste, H., and Volkow, N.D. (2018). β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proceedings of the National Academy of Science,115(17), 4483–4488. DOI/10.1073/pnas.1721694115.
Xie L, Kang, H., Xu, Q., Chen, M.J., Liao, Y., Thiyagarajan, M., O’Donnell, J., Christensen, D.J., Nicholson, C., Illif, J.J., Takano, T., Deane, R., and Nedergaard, M. (2013). Sleep drives metabolite clearance from the adult brain. Science 342(6156), 373–377. DOI: 10.1126/science 1241224




