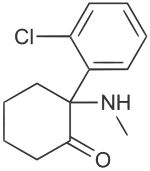
Ketamine
Rediscovering Ketamine
Uncovering new uses for an old medicine.
Posted April 25, 2024 Reviewed by Michelle Quirk
Key points
- Ketamine was approved by the FDA as a general anesthetic more than 70 years ago.
- Ketamine is also a rapidly acting antidepressant.
- Ketamine stimulates neuroplastic changes in the brain.
Coauthored by Rachel Wilkenson, MD
Ketamine was synthesized in the 1960s by scientists at Parke-Davis Laboratories while they were searching for a general anesthetic to replace phencyclidine, an older medicine that caused confusion and agitation when people awoke from surgery. Phencyclidine is also known as PCP. A series of chemicals similar to PCP, but slightly altered, were created. One of these chemicals produced excellent anesthesia and was thus selected for human trials. It was named ketamine.
Ketamine was first administered to a human in 1964. The patient responded very well. Subsequently, patients treated with ketamine reported feeling as if they were floating in space or had no feeling in their arms or legs. When one scientist described this reaction to his wife, she suggested the medicine was a “dissociative anesthetic” and this label stuck.
Ketamine was first patented in 1963 in Belgium, where it was used as a veterinary anesthetic, and in 1966 in the United States where it was used for both animal and human anesthesia. Ketamine became available by prescription under the name Ketalar in 1969.
The U.S. Food and Drug Administration (FDA) approved ketamine in 1970, the same year the Controlled Substances Act (CSA) was passed. The CSA created five schedules of controlled substances. Then, during the Vietnam War, reports of ketamine abuse emerged, resulting in this medicine being placed in Schedule III. This positioned ketamine in a less restrictive category than the attention-deficit/hyperactivity disorder (ADHD) medicines Ritalin and Adderall, but in a more restrictive group than the sleep medicine Ambien and the anti-anxiety medicine Xanax.
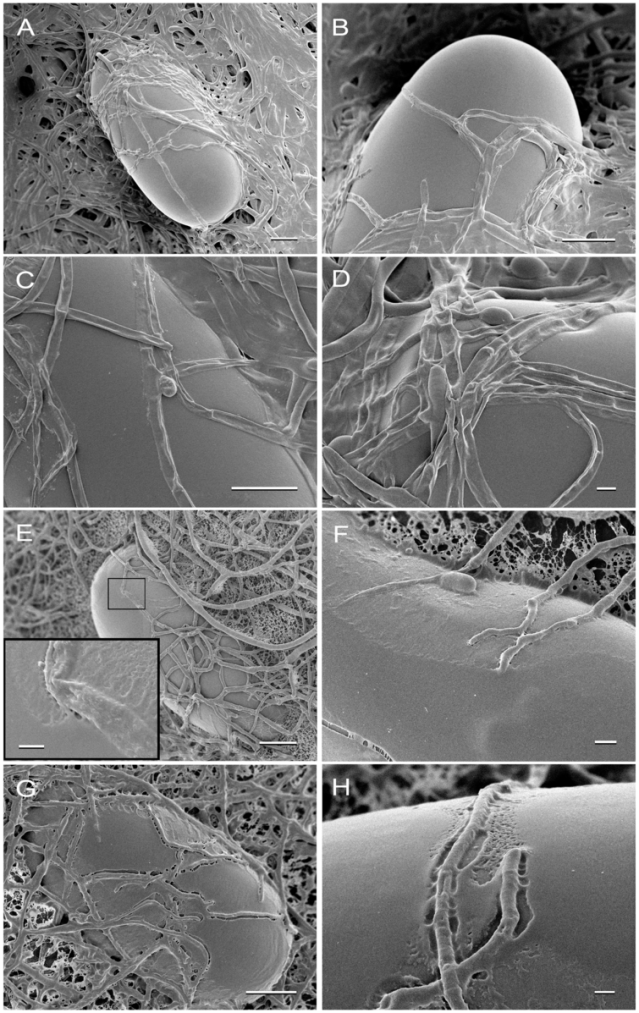
Recently, ketamine was discovered to occur in nature. While exploring treatments for parasites, scientists in Brazil found that the fungus Pochonia chlamydosporia destroys roundworms. They then took this fungus back to their lab and found it produces ketamine.
Ketamine as a rapid antidepressant
In 2000, researchers at Yale published a study demonstrating that a single dose of ketamine administered intravenously provided antidepressant effects within hours. Subsequent studies confirmed the rapid antidepressant effects of this medicine in treatment-resistant depression and bipolar depression.
A decade later, Brazilian researchers discovered that a very low dose of ketamine (10 mg) administered under the tongue every two to seven days was effective for treatment-resistant unipolar and bipolar depression. These studies inspired further investigation into other potential therapeutic uses for ketamine.
Other potential uses of ketamine
Following the demonstration that ketamine is effective as a treatment for mood disorders, subsequent studies found ketamine could help patients with suicidal ideation, social anxiety, generalized anxiety, obsessive-compulsive disorder, posttraumatic stress disorder, substance use disorders, eating disorders, and borderline personality disorder. Although the FDA has not yet approved ketamine for the treatment of these disorders, the FDA's guidelines allow health care providers to prescribe a medicine “off label” if it has been approved for one indication and is judged by the provider to be medically appropriate for their patient for other indications. This is common in the United States where close to one-third of all prescriptions for common medicines are written for off-label use.
Ketamine’s neuroplastic effects
How can one medicine provide such wide-ranging benefits? Ketamine's therapeutic effects have been attributed to this medicine's ability to induce neuroplastic changes in the brain.
Neuroplasticity refers to the brain’s ability to reorganize connections, known as synapses, between neurons. Ketamine stimulates the creation of new synapses in the brain, triggers the growth of new neurons (a process known as neurogenesis), and restores insulation on damaged neurons (referred to as re-myelination). Ketamine produces these effects by increasing a protein known as brain-derived neurotrophic factor (BDNF).
Dose and route of administration
Important questions to address when utilizing ketamine include how much is effective and how it should be administered. Reports in the popular press, such as those describing the unfortunate death of Matthew Perry, highlight the danger of using excessive doses of ketamine. As most medical students are taught, the only difference between a medicine and a poison is the dose. This aphorism highlights the importance of employing proper doses of ketamine and also carefully considering the route of administration.
Ketamine’s dose-response curve exhibits an inverted U-shape. This means that both low doses and high doses of ketamine provide little if any benefit. Intermediate doses, however, are effective. Interestingly, this U-shaped dose-response curve parallels both clinical observations of response and levels of BDNF. In a study performed in mice, researchers at Vanderbilt found that while lower doses of ketamine induced rapid antidepressant effects and increased levels of BDNF, higher doses failed to produce antidepressant effects and generated lower levels of BDNF.
The route of administration influences ketamine’s effects. When used intravenously, 100 percent of the medicine enters the bloodstream, but when taken sublingually (under the tongue), only about 30 percent of the medicine reaches the bloodstream. The term used to describe the amount of medicine entering the bloodstream via various routes is bioavailability, and the bioavailability of ketamine varies widely depending upon the route of administration.
Advantages of low-dose sublingual vs. higher-dose intravenous ketamine
Low-dose sublingual ketamine provides several benefits over higher-dose intravenous ketamine. These include the following:
- Fewer side effects. Lower doses of ketamine are associated with fewer side effects than higher doses.
- Lower cost. Low-dose sublingual ketamine is relatively inexpensive. The sublingual form is compounded at specialty pharmacies into troches (orally dissolving tablets). An average cost to the patient for a one-month supply of sublingual ketamine is $15 to $30. This compares favorably with the expense of intravenous infusions, which can run into the hundreds or thousands of dollars.
- Daily dosing. The daily administration of low-dose ketamine provides frequent stimulation of the nervous system to grow and repair. This is in contrast to the intermittent use of intravenous ketamine.
- Convenience. Low-dose sublingual ketamine can be safely self-administered at home without the need for medical supervision in a clinic setting, which is needed with intravenous ketamine.

The future of ketamine
Ketamine exhibits enormous potential as a treatment for psychiatric disorders. While additional studies are needed to elucidate additional applications, optimal dosage range, and preferred route of administration, its potential to alleviate suffering in those who have struggled to find effective treatments for their mental health maladies is likely to continue driving research.
Rachel Wilkenson, MD, is an integrated psychiatrist who has dedicated her career to helping the underserved. She is passionate about whole-body health and blending scientific rigor with the use of natural compounds to enhance recovery from severe treatment-resistant illness.
References
Domino, Edward F., and David S. Warner. "Taming the ketamine tiger." The Journal of the American Society of Anesthesiologists 113.3 (2010): 678–684.
Ferreira, Sebastiao Rodrigo, et al. "Ketamine can be produced by Pochonia chlamydosporia: an old molecule and a new anthelmintic?" Parasites & Vectors 13 (2020): 1–9.
Hull, Thomas D., et al. "At-home, sublingual ketamine telehealth is a safe and effective treatment for moderate to severe anxiety and depression: findings from a large, prospective, open-label effectiveness trial." Journal of Affective Disorders 314 (2022): 59–67.




